WELCOME TO FOREFRONT SYSTEMS
Regulatory & Quality Consultants to the Medical Device, Personal Protective Equipment (PPE), Pharmaceutical and Food Contact Industries.
Please contact us if you require consultancy support on any aspect of developing and taking medical device, PPE, Pharmaceutical and Food Contact products and technologies through to market. We would welcome the opportunity to discuss your needs in a confidential manner and propose ways in which we could work together to provide you with the best possible solution. Find more information of our company and services, contact detail:
E-Mail :
admin@forefront-syst.com , nipa.forefront@gmail.com
Mobile : +66 89 133 6252 , +66 80 471 5255

About Us
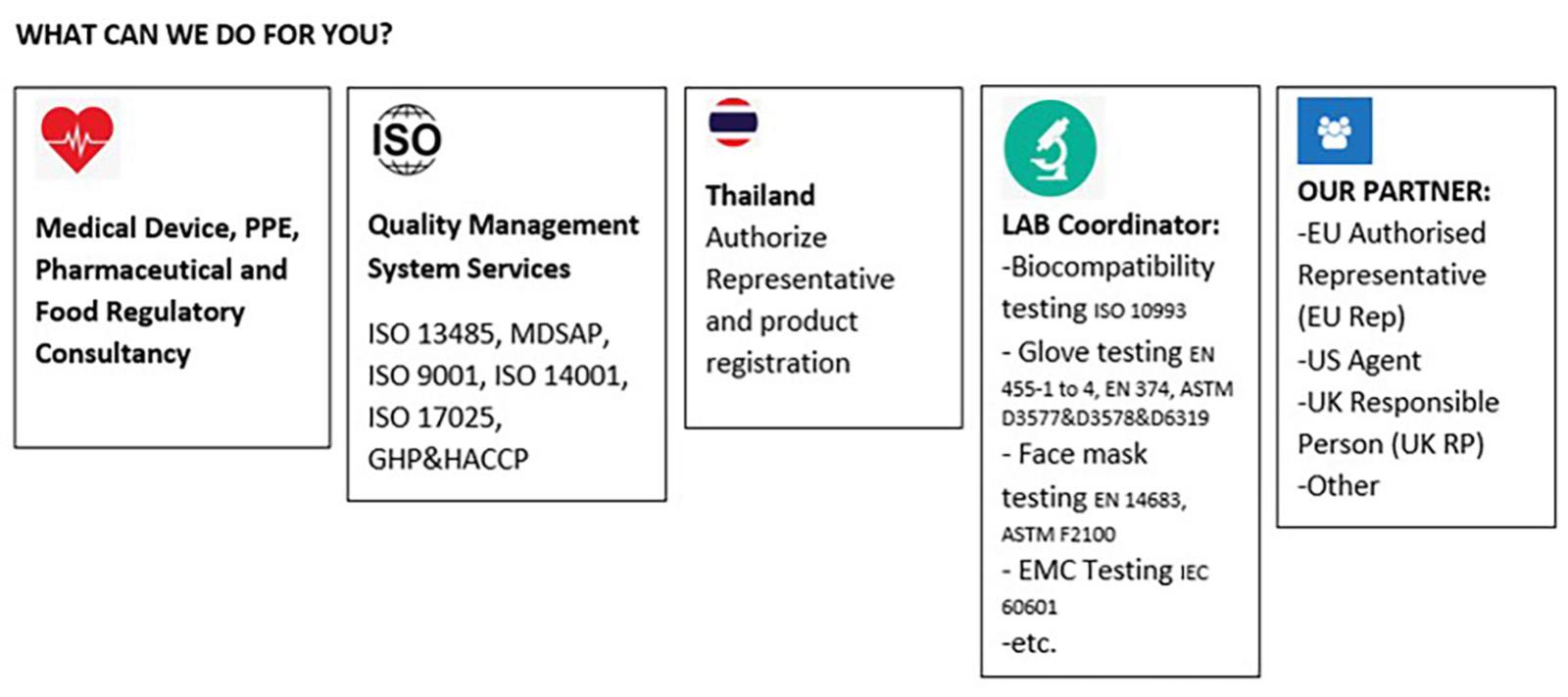
FOREFRONT SYSTEMS provides expertise for all aspects of the medical device, PPE and general products regulatory approval cycle. Our all-inclusive regulatory support includes assistance with EU Technical Files, 510K submission, UK Conformity Assessed and quality management system services, including support for the set-up and organisation of ISO 13485, MDSAP and ISO 9001 quality systems. We also provide consultancy for start-ups importer, or MNC Manufacturer who would like to set up business in Thailand to ensure compliance with all the current and relevant rules and regulations.
Established in July, 2017 and first registered in 2020 as Thailand Business, we provide consulting services to a wide range of businesses in Thailand and the globe.
Meet our team
We have group of experienced independent professionals and specialists in each product category to provide strategic advice.
Founder
Over 20 years’ experience working in Quality Management with much of her time spent working in the Medical Device industry, with 15 years of Medical Device Regulatory. Wide-ranging experience in the Medical Device Industry and Medical Device Regulatory Affairs. Involved in numerous successful CE marking projects ranging from class III to self-registration class I devices for European submissions along with US, UK, Canada, Australia, Singapore, Malaysia, China and Thailand registration and submission. Implemented, managed and audited numerous ISO 9001, ISO 13485, US 21 CFR 820, JPAL Quality Management Systems. Qualified Internal and Supplier Auditor.

Educational Background :
Master of Business Administration
B.Sc. of Chemistry
B.Sc. of Engineering /department production of engineer
FOREFRONT SYSTEMS has a range of trusted global partners to support clients who need the regulatory and quality compliance required to bring medical device products to market in the following territories: